Digital Health
Digital Health Technologies (Health IT, Data Interoperability, Health AI, Healthcare Digital Transformation) have played a central role in the scaling up of medical/health information exchange across organizational boundaries—accelerating the use of telemedicine and facilitating innovative digital health solutions to ensure more advanced and effective care delivery across the healthcare ecosystem.
 In recent years, we have observed remarkable growth and support to digitize healthcare solutions for all constituents in the healthcare ecosystem—from patients who are empowered to take ownership of their health to telehealth, which allows for lower cost and decentralized care, to robotic, precise surgeries.
In recent years, we have observed remarkable growth and support to digitize healthcare solutions for all constituents in the healthcare ecosystem—from patients who are empowered to take ownership of their health to telehealth, which allows for lower cost and decentralized care, to robotic, precise surgeries.
The FDA recognized this growing healthcare sector and established the Digital Health Center of Excellence (DHCoE) whose purpose is to:
- Connect and build partnerships to accelerate digital health advancements
- Align regulatory approach via Software as a Medical Device (SaMD) standards (such as IEC 62304) to harmonize international regulatory expectations and industry standards
- Strategically advance science and evidence for digital health technologies
- Innovate regulatory approaches to provide efficient and least burdensome* oversight while meeting the FDA standards for safe and effective products.
* The FDA defines the term “least burdensome” as a successful means of addressing a premarket issue that involves the most appropriate investment of time, effort, and resources on the part of industry and the FDA.
Source: Digital Health Center of Excellence | FDA
In addition, the Centers for Medicare & Medicaid Services (CMS) continues to build on its roadmap to improve interoperability and health information access for patients, providers, and payers. New policies were recently created that require providers and payers to implement Application Programming Interfaces (APIs) to improve the electronic exchange of healthcare data between payer and provider, which allows the ability to share more accurate information with their members/patients.
At LBG, we work diligently to stay current on the ever-changing landscape of the life sciences. Our team is comprised of subject matter experts with significant expertise in software medical device development who specialize in Market Assessment, Product Differentiation, Positioning and Messaging, Technology/Risk Assessments and Development, Gap Analysis, Usability Studies, Global Compliance/Regulatory Strategies, TPP and Product Definition (PRD), Verification and Validation (CSV), Design Control planning per FDA 21 CFR 820 and ISO 13485, Design Process planning per IEC 60601 (medical device), IEC 62304 (software as a medical device), and ISO 14971 (hazard/risk analysis), Lean UX and Agile R&D, Go-To-Market, Commercialization Strategies, Digital Patient Experience Innovation, Data and Code Standards, including HL7, LOIN, DICOM, CDISC, CDA, CPT, DRG, SNOMED, NDC, HCPCS, RxNORM, IHTSDO, FHIR, and DirectTrust, Quality Metrics and Reporting system, including HEDIS and HCAHPS, and Privacy and Security Compliance Assessment and Remediation, including the implementation of the HIPAA privacy rule and technical and administrative controls.
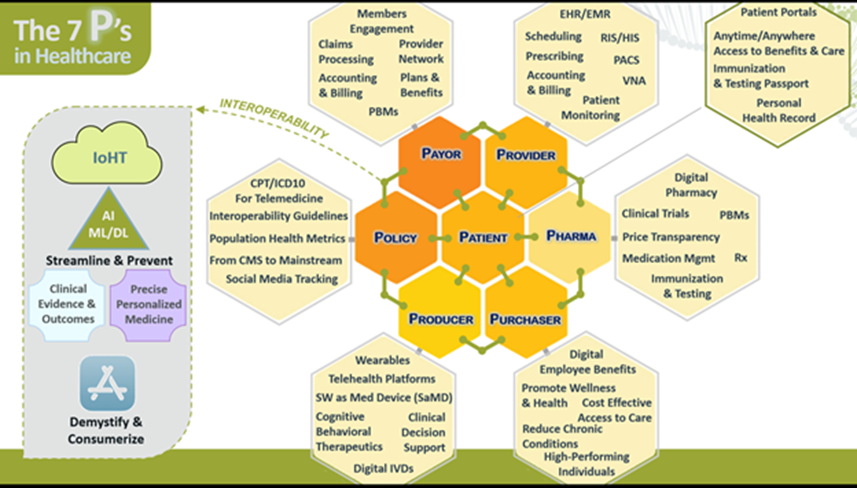
In addition, our core services support Health IT development efforts, including the following:
- Functional Product Development Expertise
- Chemistry, Manufacturing and Controls (CMC)
- Drug Discovery
- Nonclinical plan development and execution
- Clinical development and clinical study execution
- Regulatory strategy and guidance
- Quality assurance
- Program Management
- Vendor Selection and Management
- Comprehensive Non-dilutive Funding Support
- Opportunity assessment and strategic outreach/positioning
- Proposal/Solution development
- Contract negotiation
- Program and contract management
- Compliance and cost accounting services
- Product development support/expertise
- Overall Agency/NGO relations
- Strategic Consulting Services
- Strategic Planning
- Financial Modeling
- Market Research
- Supply Chain Network Development & Optimization
- Market Access/Launch
- Due Diligence
- Technology Scouting
For more information about how we can support your Health IT efforts, please schedule an appointment to speak with one of our specialists today.